Get a firm grasp on organic chemistry. Successfully studying organic chemistry means getting to know the elements of the periodic table and the important facts that highlight the fundamentals of organic chemistry. This Cheat Sheet shows it all.
>
>
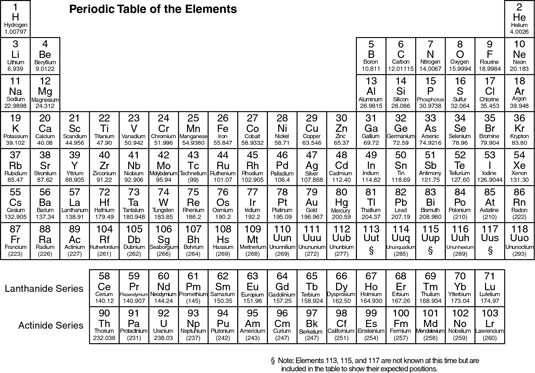
Periodic Table of Elements
Studying the elements of the periodic table is vital for understanding organic chemistry. So that you don't have to memorize each element, they're grouped together by their properties.
>
>
>
Important Concepts of Organic Chemistry
When you're studying organic chemistry, keep these helpful reminders close by because they highlight some of the most important concepts you'll need to understand organic chemistry:
Electronegativity increases as you go up and to the right in the periodic table.
In reaction mechanisms, arrows show the movement of electrons; the tip of the arrow points to where the electrons are going.
Resonance is a stabilizing feature of molecules; molecular stability generally increases as the number of resonance structures increases.
Bronsted-Lowry acids are proton donors; Bronsted-Lowry bases are proton acceptors.
Strong acids have weak (stable) conjugate bases.
Conformation refers to the way a molecule folds itself in three-dimensional space based on the rotation around carbon-carbon single bonds; configuration (such as R or S configuration of a chiral center or cis or trans configuration of double bonds) refers to the specific orientation of atoms, which can change only through a chemical reaction.
Only chiral molecules have enantiomers; enantiomers rotate plane-polarized light in equal and opposite directions.
Molecules with chiral centers that have a plane of symmetry are called meso compounds; meso compounds are achiral.
In order to have diastereomers, molecules generally have to have two or more chiral centers.
Most organic reactions are driven by an electron-rich species (a nucleophile) attacking an electron-poor species (an electrophile).
Double bonds are stabilized by alkyl substituents.
Tertiary carbocations are more stable than secondary carbocations; secondary carbocations are more stable than primary carbocations. Allylic carbocations and benzylic carbocations are about as stable as secondary carbocations.
Triple bonds are shorter than double bonds; double bonds are shorter than single bonds.
Electrophiles are Lewis acids (electron acceptors); nucleophiles are Lewis bases (electron donors).
Weak bases are good leaving groups; strong bases are bad leaving groups.
Nucleophilicity generally parallels basicity. Typically, strong bases are also good nucleophiles.
Primary halides undergo SN2 substitution; tertiary halides undergo SN1 substitution.
Aromatics have 4n + 2 pi electrons; anti-aromatic compounds have 4n pi electrons.
Substituents on aromatic rings with lone pairs on the ring-attaching atom are ortho-para directors.
>
>
dummies
Source:http://www.dummies.com/how-to/content/organic-chemistry-1-workbook-for-dummies-cheat-she.html
No comments:
Post a Comment